Abstract
Background: Early encouraging results from pharma sponsored phase 2 studies on treatment with autologous T-cells expressing anti-CD19 chimeric antigen receptor (CAR T-cells) in relapsed/refractory (R/R) aggressive B non-Hodgkin lymphomas are emerging. The outcome of patients reported in these studies compares favorably with historical data for patients treated with current standards of care. However, centralized manufacturing of these cells by pharma industry after commercialization may cause enormous logistical and financial burden to distant treating centers, especially those located outside the US. We report here our initial experience with local production of CD19-directed CAR T-cells and clinical results of young adult patients treated at Sheba Medical Center.
Methods: A phase 1b/2 study of anti-CD19 CAR T-cell therapy in pediatric and young adults with B-cell Malignancies (NCT02772198) is now underway. Patients, age 1-50 years, with R/R B-cell malignancies and adequate organ function are eligible. CD19 expression on at least 70% of malignant cells is required. The approach uses a CAR construct that is composed of an anti CD19 single-chain Fv, FMC63, CD28 co-stimulatory and CD3-zeta intracellular domains. The CAR, which was designed by the NCI, is transfected by retrovirus into bulk-stimulated autologous peripheral blood (PBL) cells. Treatment includes lymphodepletion with fludarabine 30 mg/m2 X 3 days and cyclophosphamide 900 mg/m2 x 1 day, followed by infusion of fresh transduced T cells after 9-10 days of culture. The primary end points are safety and best overall response rates (ORR), documented 28 days after infusion.
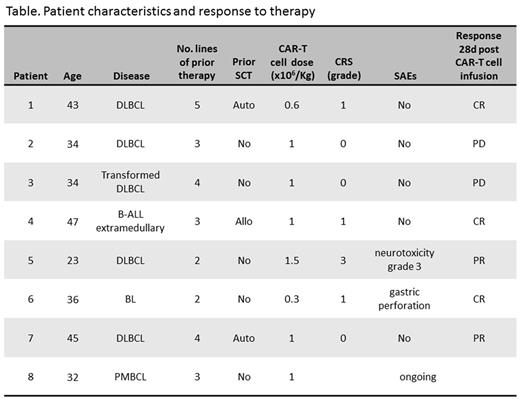
Results: As of November 2016, 8 adult patients were enrolled. The characteristics of the patients and their status following treatment are listed in the table. Briefly, 7 of them were male. Median age was 36 years (range 23-47). Four patients were diagnosed with de-novo diffuse large B cell lymphoma (DLBCL), 1 - transformed DLBCL, 1- primary mediastinal B cell lymphoma, 1 - extramedullary relapse of B-acute lymphoblastic leukemia (ALL), and 1 - Burkitt lymphoma (BL). Median number of prior therapies was 3 (range 2-5). All patients were refractory to their last therapy, including 3 cycles of blinatumomab given to the patient with B-ALL. Three had prior stem cell transplantation (1 allogeneic and 2 autologous). Patients received CAR T-cells in a dose range of 0.3 -1.5 x 106/kg, median 1 x 106/kg. The number of CAR-positive blood cells, detected by qPCR on DNA of PBL mononuclear cells, peaked between 7-14 days. Four patients developed cytokine release syndrome (CRS): 3 patients had grade 1, while one patient had concomitant grade 3 CRS and neurologic toxicity (encephalopathy and seizures). This patient was treated with tocilizumab and high dose dexamethasone, achieving complete recovery. The patient with BL had gastric sealed-perforation at the site of tumor due to therapy related necrosis, and was successfully managed with supportive care only. Seven patients are evaluable for response. At day 28 after infusion of CAR T-cells the ORR was 70% (5/7, 3 in complete response and 2 with partial response, see table) and all responses are ongoing at a median follow up of 2.5 months (range 0.7-8.4). The CAR T-cell therapy was used as a bridge to allogeneic transplantation in responding patients. Allogeneic transplantation was performed in two patients and is also scheduled for the other three responders. One patient died with progressive disease. No deaths were attributed to therapy. Enrollment of patients to this program continues.
Conclusion: Commercialization of CAR T-cell technology is inevitable and appears to be very expensive. Our preliminary results show that in-house production of CD19 directed CAR T-cells and treatment of patients with R/R aggressive B-cell malignancies using this approach in the same center is feasible and effective. Performing this entire approach in one center within a local region abrogates the need for cryopreservation and shipment of cells, and definitely reduces costs involved.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal